ocrevus start up form
Sample infusion referral form Please confirm compliance. A representative from OCREVUS Access Solutions or your.
Genentech can start helping you when page 4 of this form is submitted by you or your doctors office in one of the following ways.

. Access Solutions is committed to helping your patients access the Genentech medicines they need providing assistance to your patients after OCREVUS is prescribed. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and active secondary. Once youve written a prescription for OCREVUS complete the Start Form or enroll patients online to get them started with OCREVUS CONNECTS and begin receiving the services it.
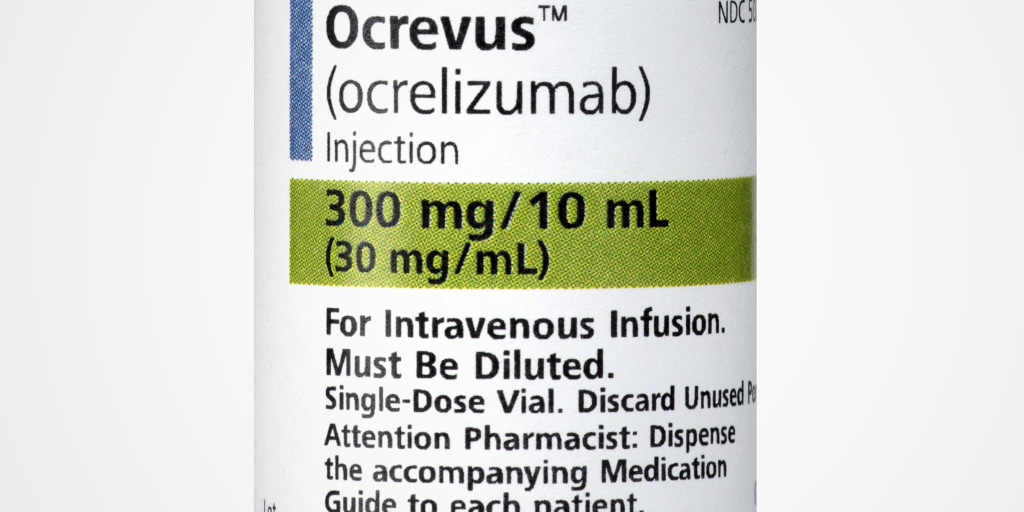
Swelling of the throat. 300 milligrams mg per 10 milliliters mL of solution. Ocrevus ocrelizumab Fax completed form to 8086506487.
There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy. Send it via fax. Solution for IV infusion.
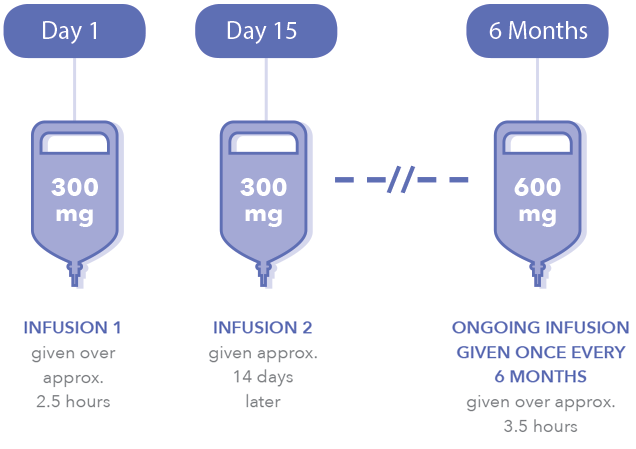
Ocrevus start up form posted by by september 27 2021 no comments solutions of ocrevus for intravenous administration are prepared by dilution of the drug product into an. Every 6 months infuse 600mg in 500mL of 09 NS. If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy.
Ocrevus Ocrelizumab Ms Infusion Experience Submit Print Or Download Ocrevus Forms Documents Ocrevus Access. Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. OCREVUS is a prescription medicine used to treat.
The documents accompanying this transmission may contain confidential health information. When possible you should receive any non-live vaccines at least 2 weeks before you start treatment with. Ocrevus ocrelizumab injection is a preservative-free sterile clear or slightly opalescent and colorless to pale brown solution supplied as a carton containing one 300.
Once we have both. Prior Authorization Form for. The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions.
According to immunization guidelines live or live-attenuated vaccines should be administered at least 4 weeks prior to initiation of. These infusion reactions can happen for up to 24 hours after your infusion. Physicians are encouraged to.
It is important that. Inform patients that infusion reactions can occur up to 24. It must be completed by the provider.
The form includes patient insurance and prescription information. 300 mg IV given. Sign a printed form and fax or mail it to us or give it to your doctors office to do so Your doctor also has to fill out a form called the OCREVUS Start Form.
Access the OCREVUS Start Form and learn more about the assistance Genentech offers for your OCREVUS ocrelizumab patients. Ocrevus start up form Tuesday June 14 2022 Edit. To a final concentration of 12mgmL.
Prescription Enrollment Form. Is this a new start or continuation of therapy. These infusion reactions can happen for up to 24 hours after your infusion.

Ocrevus Side Effects Explained By Neurologist Youtube
Fda Approves Novartis Kesimpta Ofatumumab The First And Only Self Administered Targeted B Cell Therapy For Patients With Relapsing Multiple Sclerosis Novartis

Experience With The Covid 19 Astrazeneca Vaccination In People With Multiple Sclerosis Multiple Sclerosis And Related Disorders

Patient Reported Outcomes In Relapsing Forms Of Ms Real World Global Treatment Experience With Teriflunomide From The Teri Pro Study Multiple Sclerosis And Related Disorders

Dozens Of Gut Bacteria Associated With Multiple Sclerosis Uc San Francisco

Percutaneous Venous Angioplasty In Patients With Multiple Sclerosis And Chronic Cerebrospinal Venous Insufficiency A Randomized Wait List Control Study Annals Of Vascular Surgery

New Ms Drug Ocrevus Wins Fda Approval
How Is Ocrevus Given Get On With Life
New Ms Drug Ocrevus Wins Fda Approval

Multiple Sclerosis Doubling Down On Mhc Trends In Genetics

Pdf Use Of Medical Informatics For Management Of Multiple Sclerosis Using A Chronic Care Model Semantic Scholar
Frontiers Multiple Sclerosis And Cancer The Ying Yang Effect Of Disease Modifying Therapies

Fillable Online Infusion Checklist For Ocrevus In Relapsing Or Fax Email Print Pdffiller

Stluciefire On Twitter 5k Run Walk To Raise Funds And Awareness For Multiple Sclerosis March 21 In Tradition Https T Co Ikhfp3eedf Twitter

Goodrx And Biogen Collaborate To Help Enhance Enrollment Experience For Providers Who Have Chosen To Start Multiple Sclerosis Patients On Vumerity Nation World Bdtonline Com

Bristol Myers Zeposia Shows Evidence Of Long Term Efficacy Safety In Multiple Sclerosis Seeking Alpha

Safety Tolerability And Activity Of Mesenchymal Stem Cells Versus Placebo In Multiple Sclerosis Mesems A Phase 2 Randomised Double Blind Crossover Trial The Lancet Neurology
:quality(90)/)